Abstract
BACKGROUND: Earlier studies based on the 4thgeneration CD19 chimeric antigen receptor-modified T cells (4SCAR19) targeting CD19-positive lymphomas have demonstrated improved partial or complete remission responses (PR or CR) even in very late-stage relapsed or refractory B cell lymphoma patients (pts). Nevertheless, many pts experienced relapses and became CD19 CART-resistant. To improve the long term efficacy, we have develop a CAR2.0 strategy to evaluate the safety and efficacy of sequential infusions of multi-target CARTs against lymphomas.
METHODS: Lymphoma pts who have exhausted all available treatment options with progressive or stable disease and life expectancy >2 months were enrolled in the study. Pts' lymphoma biopsies were immunostained for various target antigens including CD19, CD20, CD22, CD30, CD38, CD70 and PSMA. The choice of CART targets was based on strong positive staining results. Autologous T cells were apheresis collected and transduced with an apoptosis-inducible, safety-engineered lentiviral CAR with the following intracellular signaling domains: CD28/CD27/CD3ζ-iCasp9 (4SCAR). Pts received cyclophosphamide/fludarabine chemotherapy conditioning 1-2 days before infusions of 1.12-2.8x106 CART cells/kg per infusion. The quality of apheresis cells, efficiencies of gene transfer and T cell proliferation, CAR T infusion dose and blood CAR copies were quantitatively documented.
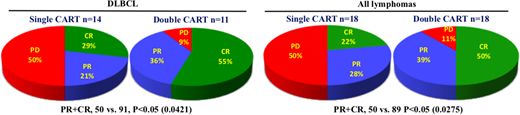
RESULTS: Total 36 pts (male and female, 1:1) were evaluated at three month follow-up time, including 25 pts with diffuse large B cell lymphoma (DLBCL), 6 with primary mediastinal B cell lymphoma (PMBL), 3 with follicular lymphoma (FL), 1 with mantle cell lymphoma (MCL), and 1 with gastric mucosal associated lymphoid tissue lymphoma (MALT). Pt characteristics include 10 pts with stage IV disease (28%), 2 after allo-transplantation (6%), 4 with bone marrow involvement (11%), 3 with CNS involvement (9%), and 3 received prior PD-1 antibody treatment (9%), and LDH was increased in 18 pts (50%). There were two treatment cohorts: 18 pts received single 4SCAR19 cells, and 18 pts received multi-CARTs targeting CD19 and an additional target, CD20, CD22, CD30, CD38, CD70 or PSMA. The choice for the additional CART was determined by individual pt's tumor antigen staining results. In the DLBCL cohort, 14 pts received single target 4SCAR19 cell infusion, at an average dose of 1.12x10e6 CART cells/kg, and resulted in 4 CR, 3 PR and 7 PD; the other 11 DLBCL pts received dual target CART infusions (CD19 plus CD20, CD22, CD38 or CD70) at an average dose of 1.32x106 CART cells/kg, and resulted in 6 CR, 4 PR and 1 PD. The three FL pts received dual target CD19+CD22 CART infusions, at an average dose of 2.55x106 CART cells/kg, and resulted in 2 CR, and 1 PR. The 6 PMBL pts, 3 received single target 4SCAR19 cell infusions, at average dose of 2.14x10e6 CART cells/kg, and resulted in 1 PR and 2 PD; the other 3 PMBL pts received dual target CART infusions (CD19+CD22, CD19+CD30), at an average dose of 2.82x106 CART cells/kg, and resulted in 1 CR, 1 PR and 1 PD. The one MCL pt received single target 4SCAR19 cell infusion, at a dose of 1.56x106 CART cells/kg, and resulted in PR. The MALT pt received double CART infusions, 2.8x106 4SCAR19 cells/kg and 2x106 4SCAR-PSMA cells/kg, and achieved PR. None of these pts developed greater than grade 2 CRS response, except for 1 DLBCL pt, who achieved CR following a grade 3 CRS. The toxicity profile of the 4SCARTs is consistent with our previous experiences with B cell acute lymphoblastic leukemia. In summary, CAR2.0 strategy has included a second CART based on immunostaining and confirmation of additional tumor specific antigen. The comparison between single CART versus double CART clearly illustrates an increased response rate for the double CART cohorts, either in DLBCL pts or in all lymphoma pts, as shown in the summary pie graphs below.
CONCLUSIONS: These early results of the multi-target 4SCAR2.0 therapy for the treatment of highly resistant lymphomas have demonstrated increased safety and improved response rate. There is clear overall clinical benefit with the multi-target CART regimen as compared with the single CD19 CART treatment. Continued follow-up will determine whether the 4SCAR2.0 therapy can obtain long term overall survival in these pts.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal